Medical devices
search
news
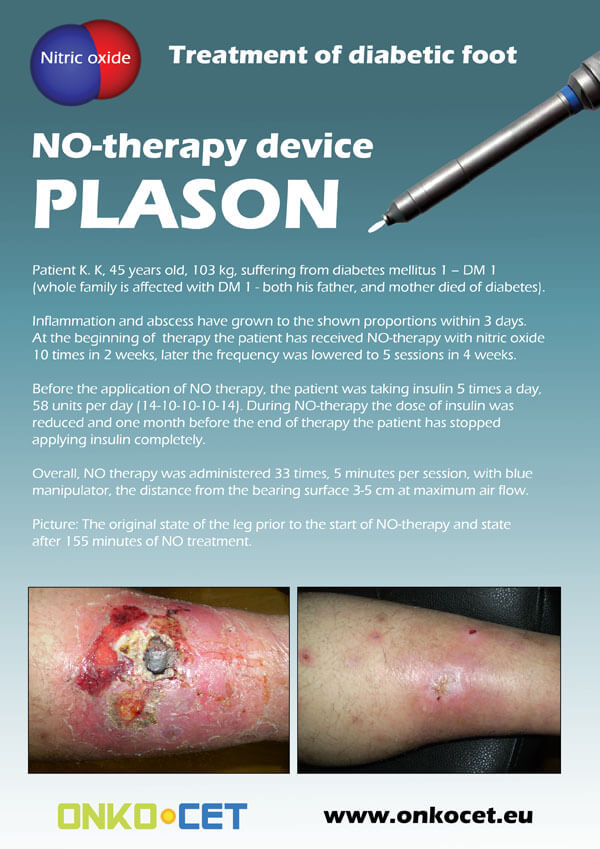
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Rikta 04/4 -2
QUANTUM MEDICINE device - Rikta 04/4 -2
 Technical characteristics
Technical characteristics
Quantity of radiators - 1+*
Area of radiating surface of the radiator - 4 sm2
Output power of radiator:
Radiator M2 - not less than 8-10W
Wavelength of impulsive infrared laser radiation - 0,890-0,910 micron/890-910 nanometers
Wavelength of pulsating broadband infrared radiation - 0,860-0,960 micron/860-960 nanometers
Wavelength of pulsating broadband red radiation - 0,640-0,740 micron/640-740 nanometers
Frequency setting - 5, 50, 1000, variable 250-1 Hz
Frequency of red light radiation - 2 Hz
Magnetic induction - 35+/-10 mTl
Time of radiation - 1, 2, 5 or 10 min.
Power supply (alternating current)
Frequency - 50/60 Hz
Power consumed from an electric network - 20W
Weight - 2,2 kgs
Overall dimensions - 245*220*95 mm.
(*possibility to apply additional emitters)
(*possibility to apply KON-1 nozzles set)
It is possible to compare Rikta 04/4 apparatus to the whole clinic! To cure more than 200 different diseases, illnesses and pains the huge hospital with the whole medical staff and expensive equipment is required. For those who has gained Rikta 04/4, there is a possibility to combine all this in one small apparatus.
The Rikta apparatus of quantum therapy has combined treatment mechanism. Rikta has several simultaneously working components:
1. Pulse infrared laser radiation(invisible) of the semi-conductor arsenide - gallium laser diode is the basic medical factor of quantum therapy. Laser radiation has a monochromaticity (bandlimitedness), spatial and time coherence and polarization and due to these properties has very strong stimulatory influence on blood circulation, membranous cellular metabolism, activates neurohumoral factors, immunocompetent systems, harmonizes hormonal factors of a metabolism.
2. Pulsating broadband infrared radiation(invisible) of semi-conductor light-emitting diodes has smaller, than laser, biological effectiveness, because of the greater spectral latitude, incoherence and nonpolarized radiation . It penetrates deeper and has a harmonizing action on the tone of central and vegetative nervous systems.
3. Pulsating broadband red radiation(visible) of semi-conductor light-emitting diodes, penetrating on slight depth, has beneficial effect, reducing intensity of inflammatory processes in a skin and hypodermic cellular tissue, especially in zones of areolar tissue.
Besides red visible light visualizes the zone of application and has weak local warming and salutary psychotherapeutic effect.
4. Magnetostaticfield turns axes of the molecular magnetic dipoles, enlarging an internal energy of molecules. It also allows to keep ionization molecules of tissues in a dissociated state. It rises the effectiveness of influence of other medical factors of quantum therapy at the molecular and cellular levels. Passing of ionized molecules and blood cells flow in a magnetic field produces their pressing to walls and turbulence of the stream, that strengthens the intensity of a saturation of tissues with oxygen and activate the process of excretion slags.
Rikta apparatus influences on human's bioactive zones with low intensity electromagnetic radiation and stimulates organism's own vital resources, improves circulation of the blood and intensifies the immune system, has anti-inflammatory and analgesic effect.
Laser ray penetrates deep into the internal organs and destroys bacterias and viruses, which are harmful for organism.
Meets the requirements of EEC
The products have EC-Certificate (Reg.-Nr. 78/705/4887) and marked with CE 0032.
The products comply with the requirements of the Medical Device Directive 93/42/EEC.