Medical devices
search
news
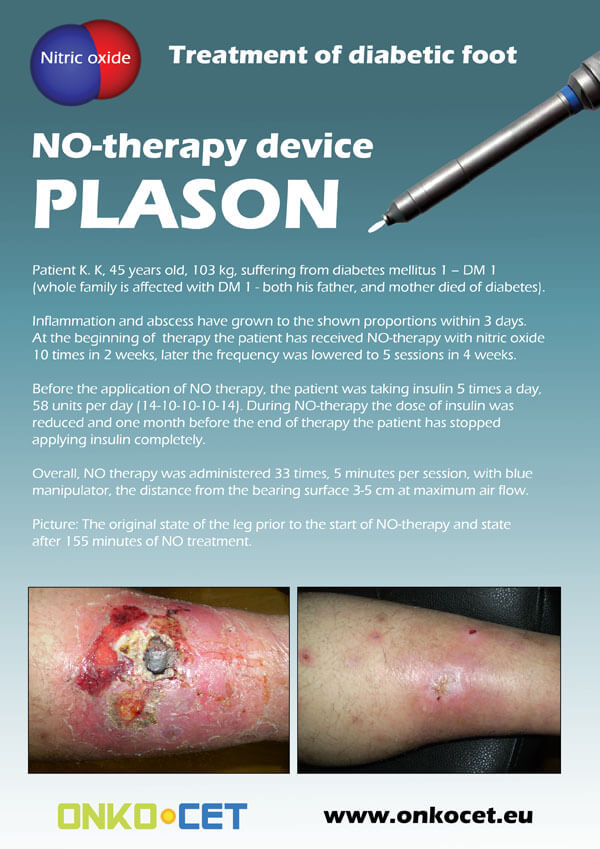
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
RTM DEVICE
RTM DEVICE
Microwave Breast Scan System - diagnostic computerized internal thermal imaging system RTM-01- RES
CLASSIFICATION
RTM-01-RES is Active Medical Device IIa class according the Rule 10 Annex IX Sect. I clause 1.4 of the Directive 93/42/EEC
INTENDED USE
The Radiometer RTM-01-RES is intended for noninvasive measuring the averaged temperature of patients’
internal tissues as well as the skin temperature.
PRODUCT SPECIFICATION
Block diagram
.jpg)
Fig. 1.The simplified scheme of the complete device set
Fig.2. The photos of the Data Processing Unit

Fig.3. Surface Temperature Sensor with nozzle (STS)
Fig.4. Internal Temperature Sensor with antenna (ITS)
Design
The antenna contacts the skin at an investigated point and receives natural electromagnetic radiation as power that is transmitted to the ITS. In the ITS the signal is amplified until it can be processed and converted to low frequencies.
After that the low frequency signal representing information on the internal temperature is processed in the DPU and the averaged temperature is displayed as a 3-digit number on the temperature indicator located on the DPU.
The signal received by the skin temperature sensor is transmitted to the DPU, where it is processed in the same way.
The skin-internal temperature switch is the sole controller on the device besides a power switch.
There are not external regulators on the device in order for simplifying a doctor’s work.
The RTM-01-RES contains the following components:
Data Processing Unit (DPU) |
Internal temperature sensor |
Antenna-applicator |
Surface temperature sensor |
Large nozzle for Surface temperature sensor |
Small nozzle for Surface temperature sensor |
Cable for DPU-PC connection |
Conductor cable |
0,16 ? Fuses |
Operating manual CD-disk RTM Diagnosis Software |
The main raw material used in the RTM-01-RES`s components is aluminium alloy.
There are no toxic chemicals used in the RTM-01-RES.
Fig. 5 The photo of the RTM-01-RES set