Biologically active supplements
search
news
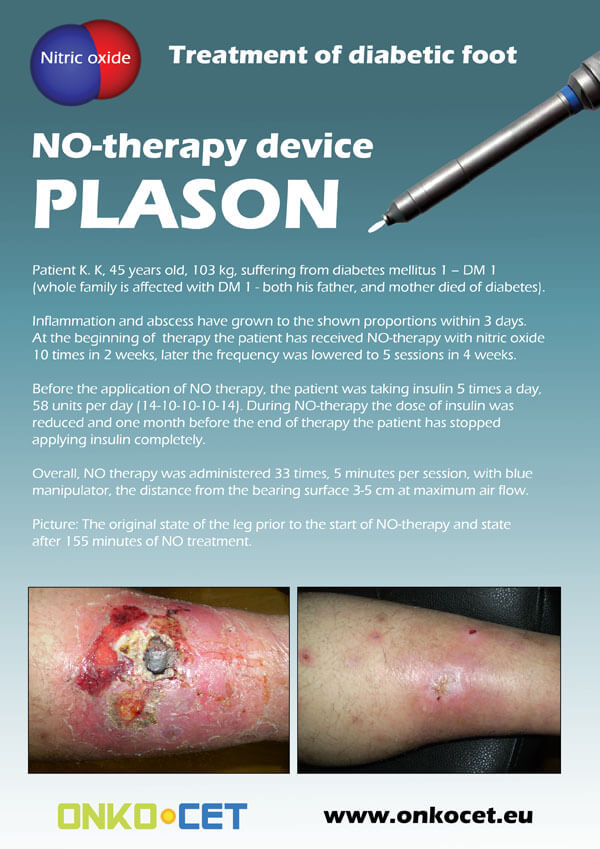
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Instructions for application
Instruction for application
 Parapharmaceutical preparation
Parapharmaceutical preparation
with immunomodulatory effect
Production form: Plastic container containing 10 gelatin capsules (0.4 g)
Ingredients:
Each gelatin capsule contains: ?-heptylglycoside-muramyl dipeptide (gl?murid) 0.1 mg; maltodextrine 400 mg.
Biological activities
An active component of Glymurid is an original synthetic derivative of group A peptidoglicane of bacterial cell wall – ?-heptylglycoside-muramyl dipeptide (glymurid). It activates significantly the main cells, which take part in immune response against infectious agents and tumors (increases cytotoxic activity of natural killer cells, monocytes/macrophages and allospecific cytotoxic T-lymphocytes; stimulates microbicidal system of granulocytes; activates T-helper function, antibody formation, and production of interleukin-1 /IL-1/, tumor necrosis factor-? /TNF- ?/, interferon-? /IFN-?/ and GM-CSF by monocytes/macrophages). Glymurid regulates also the production of other key cytokines (IL-2, -3, -4, -5, -6, -8, -10, -12, -13, IFN-?, TNF-?) and exerts either anti-inflammatory or pro-inflammatory effects depending on initial immune reactivity of the body and functional state of different components of the immunity. Glymurid causes significant increase of body resistance against tumors and bacterial, viral, mycosal infections. Besides, it stimulates regeneration of damaged tissues.
Indications
- prevention of secondary immune deficiency, infectious and tumor diseases;
- complex treatment (alone with medicines) of different diseases, particularly viral infections (including hepatitis B and C), accompanied with insufficiency of the immunity;
- prevention of infectious complications of surgical operations;
- prevention and correction of hematological and immune disorders in cancer patients undergoing radial and (or) cytostatic therapy;
- prevention of metastases and recidivations of tumors after surgical operations;
- life prolongation in patients suffering from neoplastic diseases,
- stimulation of regeneration of damaged tissues.
Contraindications
Contraindications have not been revealed
Special recommendations
Before administration in pregnant women, consult with a doctor
Side effects
Slight transitory tendency to increase granulocytes number in the peripheral blood has bean observed in several patients.
Mode of application and dosage
1-2 capsules daily or every other day for 5-20 days are recommended for activation of specific and nonspecific immunity (the mean course dosage – 10-20 capsules). To maintain the effective antitumor and anti-infective immunity, the course is to be repeated in 6 months.
In cases of prolonged Glymurid application (more than 30-40 days), intervals between each application of the preparation should be increased to 3-4 days.
For prevention of infectious complications of surgical operations, use 1 capsule daily during 5 days just before surgery and 1 capsule daily for 10-15 days after it.
For prevention of recidivations and metastases after surgical removal of primary tumor, beside the above perioperative course, the additional postoperative course (2 capsules daily every other day for 30 days) is to be administered after 1-month pause.
For complex treatment of acute and chronic hepatitis B and C, use 2 capsules every other day during 3 months. Second and third similar courses of Glymurid are to be administered after 2-week pauses.
Storage
Keep tightly closed and store in cool dry place.